Syphilis in Colorado - Provider Information
Last updated Dec. 17, 2025

Bicillin L-A Recall
Bicillin L-A shortage letter — July 16, 2025
HAN Advisory — Bicillin L-A recall — July 16, 2025
We wanted to make you aware of a new voluntary recall by King Pharmaceuticals LLC., a subsidiary of Pfizer, of specific referenced lots of Bicillin L-A (Penicillin G Benzathine Injectable Suspension). Please reach out (Bicillin Access Program 303-692-6226) if your practice received affected doses through the Bicillin access program.
Pfizer is initiating this recall due to particulates identified during visual inspection. The lots included are listed below.
| Carton NDC | Syringe NDC | Lot Number | Expiration Date YYMMDD | Strength | Configuration/ Count |
| 60793-701-10 | 60793-701-02 | GL2954 HP6222 HP6228 HP6232 HR9967 HJ3235 LT5190 | 270131 270131 271031 270930 270531 260930 270930 | 1,200,000 units/ 2 mL | 10 (2 mL) syringes per carton, 24 cartons per shipping case |
| 60793-702-10 | 60793-702-04 | GT2598 GT2599 HR9969 HK2909 HR9984 | 260930 260930 270430 270228 270831 | 2,400,000 units/ 4 mL | 10 (4 mL) syringes per carton, 24 cartons per shipping case |
Colorado strengthens protections from syphilis for pregnant people and babies
Regulation 12 of 6 CCR 1009-1: Epidemic and Communicable Disease Control went into effect January 14, 2025. This new regulation requires licensed medical professionals and all health care facilities and providers, like hospitals, urgent care clinics, community health clinics, freestanding emergency departments, medical offices, and correctional facilities, to offer additional services to stop the spread of syphilis in the state, particularly among those who are pregnant.
The rule requires all health care settings and medical providers who care for anyone who is pregnant to offer syphilis testing. Testing is required at specific points throughout pregnancy, including:
- During the first trimester of pregnancy (between 1-12 weeks), or at the patient’s first prenatal visit.
- During the third trimester (between 28-32 weeks).
- At the time of delivery.
- When there is a miscarriage after 20 weeks or stillbirth.
Labs are required to report all syphilis test results, both positive and negative, to CDPHE.
In the United States and Colorado, syphilis cases have been sharply increasing. This page includes resources for health care providers, including new Colorado screening guidelines.
Syphilis is a one-day reportable condition
In accordance with Regulation 6 CCR 1009-1 all positive and negative syphilis labs, including rapid/point of care testing, must be reported to the Colorado Department of Public Health and Environment within one working day. Both the provider and laboratory are mandated to report.
How to report:
- Contact Juan Lopez-Reyes, CDPHE Syphilis Case Ascertainment Unit Supervisor, by phone at 303-829-4263 or by email at CDPHE_STI_HIV_VHEP_DiseaseReporting@state.co.us.
- Fax a confidential morbidity report (CMR) to CDPHE Laboratory Surveillance at 303-782-5393.
- The National Network of STD Clinical Prevention Training Center offers free case consults for providers.
- Syphilis: A Call to action ECHO series slides and recordings
- CDPHE can deliver treatment to patients - see Field Delivered Therapy.
- Need help getting free Bicillin? See Bicillin access information.
| Sexually active people aged 15-44 years |
|
| Those who are pregnant |
|
| Any person being evaluated for a sexually transmitted infection |
|
| Men who have sex with men (MSM) |
|
| Transgender and gender-diverse people |
|
| People living with HIV |
|
| Neonates |
|
Specialty specific guidance
Be aware of neurosyphilis, ocular syphilis, and otosyphilis. These may occur at any stage of a syphilis infection.
- Screen for neurologic, visual, and cochleo-vestibular symptoms and signs in patients at risk for syphilis or newly diagnosed with syphilis.
- Screen patients for syphilis if they present with neurologic, visual, or cochleo-vestibular complaints.
- Conduct a careful neurological exam, including an evaluation of all cranial nerves, for patients with symptoms of neurologic, visual, or cochleo-vestibular dysfunction and reactive non treponemal and treponemal serology.
- Conduct an immediate ophthalmologic evaluation for patients with syphilis and ocular complaints.
- Evaluate and manage patients with syphilis and cochleo-vestibular symptoms in collaboration with an otolaryngologist.
Treatment for neurosyphilis, ocular syphilis, and otosyphilis should be managed according to CDC’s 2021 STI Treatment Guidelines.
Neurosyphilis
Neurosyphilis is a result of invasion of the central nervous system by Treponema pallidum, which can occur at any stage of syphilis. It is unknown whether certain T. pallidum strains are neurotropic. Early neurologic clinical manifestations (e.g., cranial nerve dysfunction, meningitis, meningovascular syphilis, stroke, and altered mental status) are usually present within the first few months or years of infection. Late neurologic manifestations (e.g., tabes dorsalis and general paresis) occur 10–30 years after infection, but can occur earlier in people who are immunocompromised.
Ocular syphilis
Ocular syphilis can occur at any stage of syphilis, with variable clinical presentations, including isolated ocular abnormalities or with neurologic manifestations. Ocular syphilis can involve almost any eye structure, but posterior uveitis and panuveitis are the most common clinical manifestations. Additional ocular manifestations may include anterior uveitis, optic neuropathy, retinal vasculitis, and interstitial keratitis. Ocular syphilis may lead to decreased visual acuity with subsequent permanent blindness.
Ocular syphilis should be managed in collaboration with an ophthalmologist. Immediate referral to an ophthalmologist is critical if ocular syphilis is suspected.
Otosyphilis
Otosyphilis is caused by an infection of the cochleovestibular system with T. pallidum and typically presents with sensorineural hearing loss, tinnitus, or vertigo. Hearing loss can be unilateral or bilateral, have a sudden onset, and progress rapidly. Otosyphilis can result in permanent hearing loss. Otosyphilis should be managed in collaboration with an otolaryngologist.
Letters
Testing for syphilis in pregnancy
Effective prevention and detection of congenital syphilis depend on identifying syphilis among those who are pregnant as early as possible.
As of January 14, 2025, all healthcare settings and medical providers who care for anyone who is pregnant are required to offer syphilis testing (Regulation 12 of 6 CCR 1009-1):
- In the first trimester of pregnancy (between 1-12 weeks), or at the patient’s first prenatal visit.
- In the third trimester (between 28-32 weeks).
- At the time of delivery.
- When there is a fetal death after 20 weeks' gestation.
- When a patient who is pregnant goes to an urgent care center or emergency room, at the intervals and events described above.
- At correctional facilities, including prisons, jails, and juvenile detention centers, at the intervals and events described above.
No birthing person or newborn infant should leave the hospital without maternal serologic status having been documented at least once during pregnancy. Any person who had no prenatal care before delivery or is considered at increased risk for syphilis acquisition during pregnancy should have the results of a syphilis serologic test documented before the patient or the neonate is discharged. A quantitative RPR is needed at the time of delivery to compare with the neonate’s nontreponemal test result.
Treatment for syphilis in pregnancy
People with a syphilis diagnosis should seek treatment as soon as possible, especially if they are pregnant, to prevent serious neonatal complications. Long-acting benzathine penicillin G therapy must be used to treat syphilis during pregnancy to prevent syphilis transmission to the infant. This therapy is extremely effective in preventing congenital syphilis, with a success rate of up to 98%. People who are allergic to penicillin should see a specialist for desensitization to penicillin.
People diagnosed with late syphilis or syphilis of unknown duration require three doses of benzathine penicillin G given one week apart. If doses are missed or given more than nine days apart, treatment must be restarted. Failure to start and complete appropriate syphilis treatment at least 30 days prior to delivery will result in a reported congenital syphilis case.
For additional information on diagnostic considerations, treatment, and follow-up, visit CDC’s Syphilis During Pregnancy page.
All infants born to a person with positive syphilis serology should have non-treponemal serology performed. Additional evaluations and treatment considerations for infants should be made following CDC’s congenital syphilis guidelines.
For information about evaluation and treatment of neonates, infants, and children for congenital syphilis, see CDC’s congenital syphilis treatment guidelines, 2021.
For resources to communicate with the public and providers about congenital syphilis, its risks, treatment, and prevention, use CDPHE’s congenital syphilis communications toolkit.
On January 16, 2024, the FDA announced that they have exercised enforcement discretion for a temporary importation and use of Extencillin (benzathine benzylpenicillin injection, powder, for suspension) to mitigate the effects of the Bicillin L-A® drug shortage. See more in this CDC letter. On June 10, 2024, Pfizer shared an update on their 2.4 million Units/4 milliliter Bicillin L-A® supply, noting that they currently have available supply. If there is sufficient supply of Bicillin L-A ® in your jurisdiction, please consider using Bicillin L-A® for all appropriate patients, per CDC's standard guidance.
Pharmacy letter about Bicillin shortage
CDC has received reports that some STD programs are currently unable to procure enough penicillin G benzathine (Bicillin L-A®) — the first-line recommended treatment for syphilis — for their jurisdictions.
During this time, programs should follow CDC's 2021 Sexually Transmitted Infections Treatment Guidelines.
Intramuscular, long-acting benzathine penicillin G (or aqueous crystalline penicillin G, in cases of ocular, otic or neurosyphilis) remains the only regimen with documented efficacy against syphilis during pregnancy and for the prevention of congenital syphilis. Those who are pregnant and allergic to penicillin should be referred for desensitization and treated with the CDC-recommended penicillin regimen. Congenital syphilis treatment guidelines are discussed on CDC’s Congenital Syphilis webpage.
Doxycycline 100mg PO BID for two weeks (for early syphilis) or for four weeks (for late latent or syphilis of unknown duration) is an alternative treatment for non-pregnant people with a penicillin allergy. Tetracycline (500 mg orally four times a day for 14 days) is another alternative. Barriers to treatment compliance should be considered.
Prioritization of treatment with Bicillin L-A® may be indicated for certain populations such as those who are pregnant, babies with congenital syphilis, people experiencing homelessness, people who use drugs, or other populations in which treatment completion may be a barrier.
Email DSTDP (stdshortages@cdc.gov) about any shortage or low inventories of Bicillin L-A® in your jurisdiction so CDC can continue to monitor this situation and provide situational awareness to FDA and Pfizer. Report any shortages to the Pfizer Supply Continuity Team at 844-646-4398 (select 1 and then select 3).
To view a list of agencies that provide syphilis testing and treatment, visit the CDPHE STI/HIV testing and treatment locator.
If you have questions about treatment during the Bicillin shortage, contact Adrianna Hervey, Surveillance and Case Ascertainment Program Manager, at adrianna.hervey@state.co.us or 720-263-0415; or Lacy Mulleavey, Prevention Field Services Program Manager, at lacy.mulleavey@state.co.us or 303-692-2674.
General information
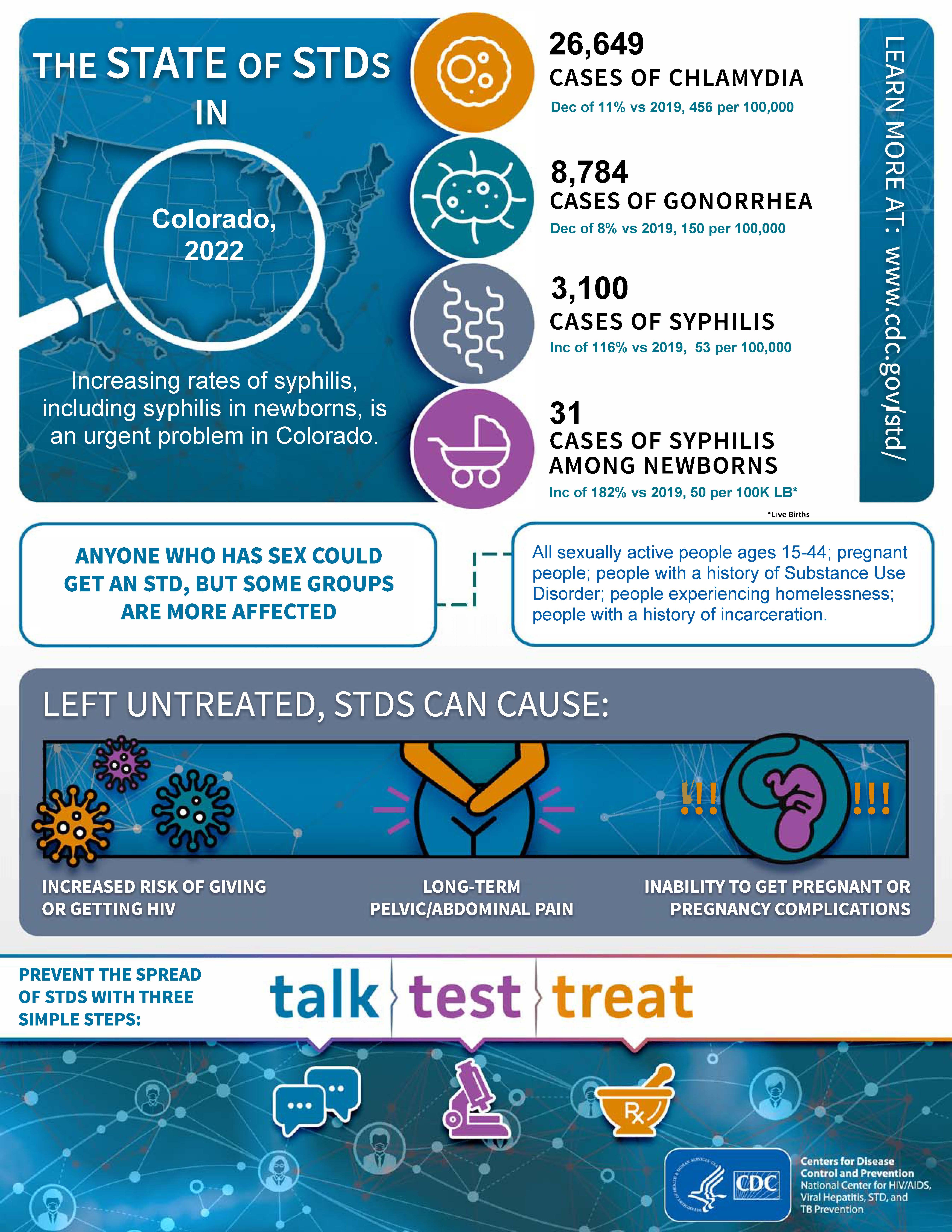
In the United States and Colorado, syphilis cases have been sharply increasing. Colorado saw a three-fold increase from 2018 to 2023. The rate of congenital syphilis has been increasing nationally and in Colorado since 2012, with a seven-fold increase in Colorado between 2018 and 2023. A total of 50 cases of congenital syphilis were reported to CDPHE in 2023. Among the cases reported in 2023, syphilis was acquired during pregnancy 28% of the time. This indicates that first trimester screening alone may be inadequate for congenital syphilis prevention. Syphilis cases among women of reproductive age) increased by a factor of seven during the same time period (2018-2023). 52% of cases among women of reproductive age had no known social factors associated with increased risk of syphilis infection. This suggests that risk-based screening may also be insufficient.
State-level statistics change weekly. Specific numbers may change as more data becomes available. However, the trend of significant increases in all stages of syphilis, including congenital syphilis, as well as complications of syphilis, has continued.
Syphilis is a sexually transmitted infection caused by the bacterium Treponema pallidum. Syphilis is divided into stages (primary, secondary, latent, and tertiary), with different signs and symptoms associated with each stage. Detailed guidance on staging of syphilis can be found on The Diagnosis, Management and Prevention of Syphilis: An Update and Review. A person with primary syphilis generally has an ulceration at the original site of infection (e.g. around genitals, anus/rectum, or mouth). These ulcers are usually (but not always) firm, round, and painless. Common symptoms of secondary syphilis include skin rash, swollen lymph nodes, and fever. The signs and symptoms of primary and secondary syphilis can be mild, and they might not be noticed. During the latent stage, there are no signs or symptoms, but the infection is present and could be progressing. Tertiary syphilis refers to gummas (soft, tumor-like growth of the tissues (granuloma) that occurs in people with late-stage tertiary syphilis, most often seen in the liver), cardiovascular syphilis, psychiatric manifestations (e.g., memory loss or personality changes), or late neurosyphilis.
Someone who is pregnant can transmit syphilis to their child during any stage of syphilis and any trimester of pregnancy. However, the risk of transmission is highest if the person has acquired syphilis recently. Syphilis during pregnancy increases adverse pregnancy outcomes including preterm birth and stillbirth. As many as 40% of babies born to people with untreated syphilis (if acquired within four years prior to delivery) will be stillborn or die in infancy. Congenital syphilis can lead to newborn and childhood illness including hydrops fetalis; hepatosplenomegaly; rashes; fevers; failure to thrive; deformity of the face, teeth, and bones; blindness; and deafness. More information can be found in the CDC Congenital Syphilis Fact Sheet.
For additional background information, see the CDC STI Surveillance Report.
Unsure how to take a complete sexual history? Use this CDC resource: A Guide to Taking a Sexual History.
Screening requires use of a standardized algorithm. Two tests are required to diagnose syphilis, a non-treponemal assay (i.e., Venereal Disease Research Laboratory [VDRL] or Rapid Plasma Reagin [RPR]) and a treponemal test (i.e., fluorescent treponemal antibody absorbed [FTA-ABS] tests, the pallidum passive particle agglutination [TP-PA] assay, etc). For more information on interpreting syphilis serologies, contact CDPHE (juan.lopez-reyes@state.co.us or adrianna.hervey@state.co.us) or the CDC-supported STD expert consultation online service.
Past history of a syphilis diagnosis and treatment can be obtained from CDPHE by contacting Juan Lopez-Reyes, CDPHE Syphilis Case Ascertainment Unit Supervisor, at 303-829-4263 or juan.lopez-reyes@state.co.us.
Syphilis screening algorithms
Serologic testing is the standard for syphilis diagnosis. Serologic testing is divided into two major groups: treponemal, which tests for specific antibodies to Treponema pallidum (the causative organism), and nontreponemal, which tests for antibodies to a cardiolipin-cholesterol-lecithin antigen. Treponemal antibody tests include the fluorescent treponemal antibody absorption (FTA-ABS), Treponema pallidum particle agglutination assay (TP-PA), enzyme immunoassays (EIAs), and chemiluminescence immunoassays (CIAs).
Non-treponemal tests include the rapid plasma reagin (RPR) test and the Venereal Disease Research Laboratory (VDRL) test. The RPR is performed on blood and the VDRL can be performed on blood or spinal fluid.
The diagnosis of syphilis by serology requires the use of both nontreponemal and treponemal tests. Hence, there are two commonly used approaches for the serologic diagnosis of syphilis — traditional and “reverse” algorithms.
Traditional and reverse screening algorithms
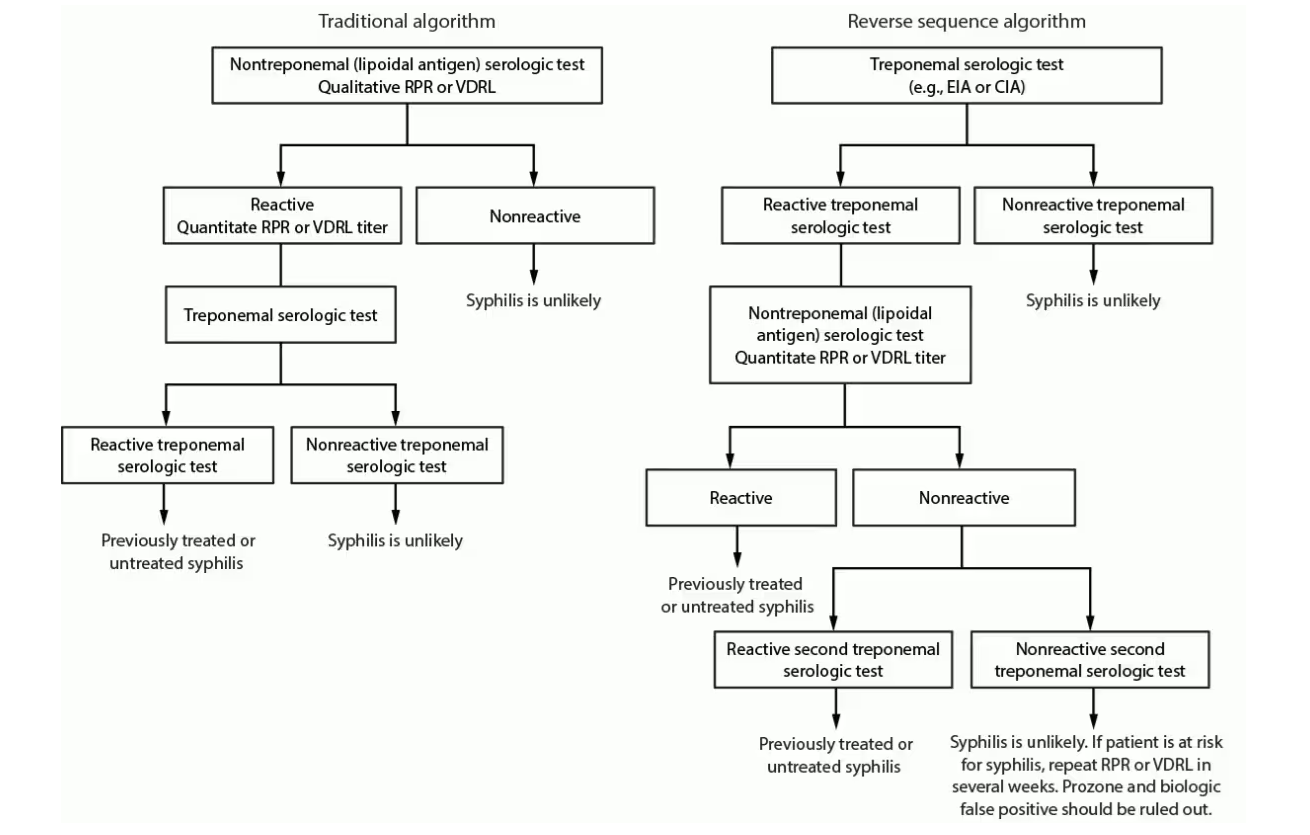
Figure from CDC Laboratory Recommendations for Syphilis Testing, United States, 2024.
False-positive nontreponemal test results can be associated with multiple medical conditions and factors unrelated to syphilis, including other infections (e.g., HIV), autoimmune conditions, vaccinations, injecting drug use, pregnancy, and older age. Therefore, people with a reactive nontreponemal test should always receive a treponemal test to confirm the syphilis diagnosis (i.e., traditional algorithm).
The majority of patients who have reactive treponemal tests will have reactive tests for the remainder of their lives, regardless of adequate treatment or disease activity.
Reverse sequence algorithm for syphilis testing can identify people previously treated for syphilis, those with untreated or incompletely treated syphilis, and those with false-positive results that can occur with a low likelihood of infection.
People with a positive treponemal screening test should have a standard quantitative nontreponemal test with titer performed reflexively by the laboratory to guide patient management decisions. If the nontreponemal test is negative, the laboratory should perform a treponemal test different from the one used for initial testing, preferably TP-PA or treponemal assay based on different antigens than the original test, to adjudicate the results of the initial test.
Major commercial lab algorithms
Additional laboratory resources:
Syphilis can be separated into four different stages: primary, secondary, early non-primary/non-secondary, and unknown duration or late syphilis. Understanding the different stages for syphilis is crucial to managing syphilis. Symptoms help understand more about the duration of the infection and the treatment needed.
Want to learn more? Visit the links to learn more about staging and treating syphilis:
- Syphilis Treatment Guidelines 2021 (CDC)
- STI Treatment Guidelines Pocket Guide (CDC)
- Syphilis - CDC Fact Sheet
- Syphilis-pocket-guide (PDF)
Need help finding syphilis testing and/or treatment? Use our Syphilis Testing & Treatment Locator. If you would like to add or update your agency information, complete the Provider Interest Form.
For help with Colorado Medicaid claims regarding syphilis testing and coverage, contact the Member Contact Center at 1-800-221-3943 / State Relay: 711.
Providers are reminded that STI laboratory testing is a covered benefit without limitations under Health First Colorado (Colorado's Medicaid program). Refer to the Laboratory Services Billing Manual for more information, including billing codes.
.